Protein structures
| Line 75: | Line 75: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:2dt6 asym r 250.jpg|120px]] |
|Kuwasako, K., He, F., Inoue, M., Tanaka, A., Sugano, S., Güntert, P., Muto, Y. & Yokoyama, S. Solution structures of the SURP domains and the subunit-assembly mechanism within the splicing factor SF3a complex in 17S U2 snRNP[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Kuwasako06-SURPDomain.pdf .] [http://dx.doi.org/10.1016/j.str.2006.09.009 Structure 14, 1677–1689 (2006)] | |Kuwasako, K., He, F., Inoue, M., Tanaka, A., Sugano, S., Güntert, P., Muto, Y. & Yokoyama, S. Solution structures of the SURP domains and the subunit-assembly mechanism within the splicing factor SF3a complex in 17S U2 snRNP[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Kuwasako06-SURPDomain.pdf .] [http://dx.doi.org/10.1016/j.str.2006.09.009 Structure 14, 1677–1689 (2006)] | ||
| Line 81: | Line 81: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:2dcp asym r 250.jpg|120px]] |
|ENTH-VHS domain At3g16270(9-135) from ''Arabidopsis thaliana''. | |ENTH-VHS domain At3g16270(9-135) from ''Arabidopsis thaliana''. | ||
López-Méndez, B. & Güntert, P. Automated protein structure determination from NMR spectra[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Lopez06a.pdf .] [http://dx.doi.org/10.1021/ja061136l J. Am. Chem. Soc. 128, 13112–13122 (2006)] | López-Méndez, B. & Güntert, P. Automated protein structure determination from NMR spectra[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Lopez06a.pdf .] [http://dx.doi.org/10.1021/ja061136l J. Am. Chem. Soc. 128, 13112–13122 (2006)] | ||
| Line 90: | Line 90: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:2dcq asym r 250.jpg|120px]] |
|Rhodanese homology domain At3g16270(175-295) from ''Arabidopsis thaliana''. | |Rhodanese homology domain At3g16270(175-295) from ''Arabidopsis thaliana''. | ||
López-Méndez, B. & Güntert, P. Automated protein structure determination from NMR spectra[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Lopez06a.pdf .] [http://dx.doi.org/10.1021/ja061136l J. Am. Chem. Soc. 128, 13112–13122 (2006)] | López-Méndez, B. & Güntert, P. Automated protein structure determination from NMR spectra[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Lopez06a.pdf .] [http://dx.doi.org/10.1021/ja061136l J. Am. Chem. Soc. 128, 13112–13122 (2006)] | ||
| Line 99: | Line 99: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:2dcr asym r 250.jpg|120px]] |
|Src homology domain 2 from the human feline sarcoma oncogene Fes. | |Src homology domain 2 from the human feline sarcoma oncogene Fes. | ||
López-Méndez, B. & Güntert, P. Automated protein structure determination from NMR spectra[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Lopez06a.pdf .] [http://dx.doi.org/10.1021/ja061136l J. Am. Chem. Soc. 128, 13112–13122 (2006)] | López-Méndez, B. & Güntert, P. Automated protein structure determination from NMR spectra[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Lopez06a.pdf .] [http://dx.doi.org/10.1021/ja061136l J. Am. Chem. Soc. 128, 13112–13122 (2006)] | ||
| Line 106: | Line 106: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:2h3t asym r 250.jpg|120px]] |
|Jurt, S., Aemissegger, A., Güntert, P., Zerbe, O. & Hilvert, D. A photoswitchable miniprotein based on the sequence of avian pancreatic polypeptide[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Jurt06a.pdf .] [http://dx.doi.org/10.1002/anie.200602084 Angew. Chem. Int. Ed. 45, 6297-6300 (2006)] | |Jurt, S., Aemissegger, A., Güntert, P., Zerbe, O. & Hilvert, D. A photoswitchable miniprotein based on the sequence of avian pancreatic polypeptide[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Jurt06a.pdf .] [http://dx.doi.org/10.1002/anie.200602084 Angew. Chem. Int. Ed. 45, 6297-6300 (2006)] | ||
| Line 112: | Line 112: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:2wvo asym r 250.jpg|120px]] |
|Hamada, T., Ito, Y., Abe, T., Hayashi, F., Güntert, P., Inoue, M., Kigawa, T., Terada, T., Shirouzu, M., Yoshida, M., Tanaka, A., Sugano, S., Yokoyama, S. & Hirota, H. Solution structure of the antifreeze-like domain of human sialic acid synthase[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Hamada06a.pdf .] [http://dx.doi.org/10.1110/ps.051700406 Protein Sci. 15, 1010–1016 (2006)] | |Hamada, T., Ito, Y., Abe, T., Hayashi, F., Güntert, P., Inoue, M., Kigawa, T., Terada, T., Shirouzu, M., Yoshida, M., Tanaka, A., Sugano, S., Yokoyama, S. & Hirota, H. Solution structure of the antifreeze-like domain of human sialic acid synthase[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Hamada06a.pdf .] [http://dx.doi.org/10.1110/ps.051700406 Protein Sci. 15, 1010–1016 (2006)] | ||
| Line 118: | Line 118: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:2agm asym r 250.jpg|120px]] |
|Aachmann, F. L., Svanem, B. I. G., Güntert, P., Petersen, S. B., Valla, S. & Wimmer, R. NMR structure of the R-module - A parallel β-roll subunit from an ''Azotobacter vinelandii'' mannuronan C-5 epimerase[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Aachmann06a.pdf .] [http://dx.doi.org/10.1074/jbc.M510069200 J. Biol. Chem. 281, 7350–7356 (2006)] | |Aachmann, F. L., Svanem, B. I. G., Güntert, P., Petersen, S. B., Valla, S. & Wimmer, R. NMR structure of the R-module - A parallel β-roll subunit from an ''Azotobacter vinelandii'' mannuronan C-5 epimerase[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Aachmann06a.pdf .] [http://dx.doi.org/10.1074/jbc.M510069200 J. Biol. Chem. 281, 7350–7356 (2006)] | ||
| Line 124: | Line 124: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:2d21 asym r 250.jpg|120px]] |
|Stereo-array isotope labeled (SAIL) maltodextrin-binding protein MBP. | |Stereo-array isotope labeled (SAIL) maltodextrin-binding protein MBP. | ||
Kainosho, M., Torizawa, T., Iwashita, Y., Terauchi, T., Ono, A. M. & Güntert, P. Optimal isotope labeling for NMR protein structure determinations[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Kainosho06a.pdf .] [http://dx.doi.org/10.1038/nature04525 Nature 440, 52–57 (2006)] | Kainosho, M., Torizawa, T., Iwashita, Y., Terauchi, T., Ono, A. M. & Güntert, P. Optimal isotope labeling for NMR protein structure determinations[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Kainosho06a.pdf .] [http://dx.doi.org/10.1038/nature04525 Nature 440, 52–57 (2006)] | ||
| Line 131: | Line 131: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1x02 asym r 250.jpg|120px]] |
|Stereo-array isotope labeled (SAIL) calmodulin. | |Stereo-array isotope labeled (SAIL) calmodulin. | ||
Kainosho, M., Torizawa, T., Iwashita, Y., Terauchi, T., Ono, A. M. & Güntert, P. Optimal isotope labeling for NMR protein structure determinations[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Kainosho06a.pdf .] [http://dx.doi.org/10.1038/nature04525 Nature 440, 52–57 (2006)] | Kainosho, M., Torizawa, T., Iwashita, Y., Terauchi, T., Ono, A. M. & Güntert, P. Optimal isotope labeling for NMR protein structure determinations[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Kainosho06a.pdf .] [http://dx.doi.org/10.1038/nature04525 Nature 440, 52–57 (2006)] | ||
| Line 138: | Line 138: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1wwq asym r 250.jpg|120px]] |
|Li, H., Inoue, M., Yabuki, T., Aoki, M., Seki, E., Matsuda, T., Nunokawa, E., Motoda, Y., Kobayashi, A., Terada, T., Shirouzu, M., Koshiba, S., Lin, Y. J., Güntert, P., Suzuki, H., Hayashizaki, Y., Kigawa, T. & Yokoyama, S. Solution structure of the mouse enhancer of rudimentary protein reveals a novel fold[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Li05a.pdf .] [http://dx.doi.org/10.1007/s10858-005-7959-z J. Biomol. NMR 32, 329–334 (2005)] | |Li, H., Inoue, M., Yabuki, T., Aoki, M., Seki, E., Matsuda, T., Nunokawa, E., Motoda, Y., Kobayashi, A., Terada, T., Shirouzu, M., Koshiba, S., Lin, Y. J., Güntert, P., Suzuki, H., Hayashizaki, Y., Kigawa, T. & Yokoyama, S. Solution structure of the mouse enhancer of rudimentary protein reveals a novel fold[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Li05a.pdf .] [http://dx.doi.org/10.1007/s10858-005-7959-z J. Biomol. NMR 32, 329–334 (2005)] | ||
| Line 144: | Line 144: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1wqu asym r 250.jpg|120px]] |
|Scott, A., Pantoja-Uceda, D., Koshiba, S., Inoue, M., Kigawa, T., Terada, T., Shirouzu, M., Tanaka, A., Sugano, S., Yokoyama, S. & Güntert, P. Solution structure of the Src homology 2 domain from the human feline sarcoma oncogene Fes[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Scott05a.pdf .] [http://dx.doi.org/10.1007/s10858-005-0946-6 J. Biomol. NMR. 31, 357–361 (2005)] | |Scott, A., Pantoja-Uceda, D., Koshiba, S., Inoue, M., Kigawa, T., Terada, T., Shirouzu, M., Tanaka, A., Sugano, S., Yokoyama, S. & Güntert, P. Solution structure of the Src homology 2 domain from the human feline sarcoma oncogene Fes[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Scott05a.pdf .] [http://dx.doi.org/10.1007/s10858-005-0946-6 J. Biomol. NMR. 31, 357–361 (2005)] | ||
| Line 150: | Line 150: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1n27 asym r 250.jpg|120px]] |
|Nameki, N., Tochio, N., Koshiba, S., Inoue, M., Yabuki, T., Aoki, M., Seki, E., Matsuda, T., Fujikura, Y., Saito, M., Ikari, M., Watanabe, M., Terada, T., Shirouzu, M., Yoshida, M., Hirota, H., Tanaka, A., Hayashizaki, Y., Güntert, P., Kigawa, T. & Yokoyama, S. Solution structure of the PWWP domain of the hepatoma-derived growth factor family[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Nameki05a.pdf .] [http://dx.doi.org/10.1110/ps.04975305 Protein Sci. 14, 756–764 (2005)] | |Nameki, N., Tochio, N., Koshiba, S., Inoue, M., Yabuki, T., Aoki, M., Seki, E., Matsuda, T., Fujikura, Y., Saito, M., Ikari, M., Watanabe, M., Terada, T., Shirouzu, M., Yoshida, M., Hirota, H., Tanaka, A., Hayashizaki, Y., Güntert, P., Kigawa, T. & Yokoyama, S. Solution structure of the PWWP domain of the hepatoma-derived growth factor family[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Nameki05a.pdf .] [http://dx.doi.org/10.1110/ps.04975305 Protein Sci. 14, 756–764 (2005)] | ||
| Line 156: | Line 156: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1u3m asym r 250.jpg|120px]] |
|Calzolai, L., Lysek, D. A., Pérez, D. R., Güntert, P. & Wüthrich, K. Prion protein NMR structures of chicken, turtle and frog[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Calzolai05a.pdf .] [http://dx.doi.org/10.1073/pnas.0408939102 Proc. Natl. Acad. Sci. USA 102, 651–655 (2005)] | |Calzolai, L., Lysek, D. A., Pérez, D. R., Güntert, P. & Wüthrich, K. Prion protein NMR structures of chicken, turtle and frog[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Calzolai05a.pdf .] [http://dx.doi.org/10.1073/pnas.0408939102 Proc. Natl. Acad. Sci. USA 102, 651–655 (2005)] | ||
| Line 162: | Line 162: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1xyj asym r 250.jpg|120px]] |
|Lysek, D. A., Schorn, C., Nivon, L. G., Esteve-Moya, V., Christen, B., Calzolai, L., von Schroetter, C., Fiorito, F., Herrmann, T., Güntert, P. & Wüthrich, K. Prion protein NMR structures of cat, dog, pig and sheep[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Lysek05a.pdf .] [http://dx.doi.org/10.1073/pnas.0408937102 Proc. Natl. Acad. Sci. USA 102, 640–645 (2005)] | |Lysek, D. A., Schorn, C., Nivon, L. G., Esteve-Moya, V., Christen, B., Calzolai, L., von Schroetter, C., Fiorito, F., Herrmann, T., Güntert, P. & Wüthrich, K. Prion protein NMR structures of cat, dog, pig and sheep[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Lysek05a.pdf .] [http://dx.doi.org/10.1073/pnas.0408937102 Proc. Natl. Acad. Sci. USA 102, 640–645 (2005)] | ||
| Line 168: | Line 168: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1vex asym r 250.jpg|120px]] |
|Pääkkönen, K., Tossavainen, H., Permi, P., Rakkolainen, H., Rauvala, H., Raulo, E., Kilpeläinen, I. & Güntert, P. Solution structures of the first and fourth TSR domains of F-spondin[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Paakkonen06a.pdf .] [http://dx.doi.org/10.1002/prot.21030 Proteins 64, 665–672 (2006)] | |Pääkkönen, K., Tossavainen, H., Permi, P., Rakkolainen, H., Rauvala, H., Raulo, E., Kilpeläinen, I. & Güntert, P. Solution structures of the first and fourth TSR domains of F-spondin[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Paakkonen06a.pdf .] [http://dx.doi.org/10.1002/prot.21030 Proteins 64, 665–672 (2006)] | ||
| Line 174: | Line 174: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1vdy asym r 250.jpg|120px]] |
|ENTH-VHS domain At3g16270 from Arabidopsis thaliana. | |ENTH-VHS domain At3g16270 from Arabidopsis thaliana. | ||
| Line 180: | Line 180: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1vd0 asym r 250.jpg|120px]] |
|Iwai, H., Forrer, P., Plückthun, A. & Güntert, P. NMR solution structure of the monomeric form of the bacteriophage λ capsid stabilizing protein gpD[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Iwai05a.pdf .] [http://dx.doi.org/10.1007/s10858-005-0945-7 J. Biomol. NMR. 31, 351–356 (2005)] | |Iwai, H., Forrer, P., Plückthun, A. & Güntert, P. NMR solution structure of the monomeric form of the bacteriophage λ capsid stabilizing protein gpD[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Iwai05a.pdf .] [http://dx.doi.org/10.1007/s10858-005-0945-7 J. Biomol. NMR. 31, 351–356 (2005)] | ||
| Line 186: | Line 186: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1vee asym r 250.jpg|120px]] |
|Pantoja-Uceda, D., López-Méndez, B., Koshiba, S., Inoue, M., Kigawa, T., Terada, T., Shirouzu, M., Tanaka, A., Seki, M., Shinozaki, K., Yokoyama, S. & Güntert, P. Solution structure of the rhodanese homology domain At4g01050(175–295) from Arabidopsis thaliana[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Pantoja05a.pdf .] [http://dx.doi.org/10.1110/ps.041138705 Protein Sci. 14, 224–230 (2005)] | |Pantoja-Uceda, D., López-Méndez, B., Koshiba, S., Inoue, M., Kigawa, T., Terada, T., Shirouzu, M., Tanaka, A., Seki, M., Shinozaki, K., Yokoyama, S. & Güntert, P. Solution structure of the rhodanese homology domain At4g01050(175–295) from Arabidopsis thaliana[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Pantoja05a.pdf .] [http://dx.doi.org/10.1110/ps.041138705 Protein Sci. 14, 224–230 (2005)] | ||
| Line 192: | Line 192: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1ukx asym r 250.jpg|120px]] |
|Nameki, N., Yoneyama, M., Koshiba, S., Tochio, N., Inoue, M., Seki, E., Matsuda, T., Tomo, Y., Harada, T., Saito, K., Kobayashi, N., Yabuki, T., Aoki, M., Nunokawa, E., Matsuda, N., Sakagami, N., Terada, T., Shirouzu, M., Yoshida, M., Hirota, H., Osanai, T., Tanaka, A., Arakawa, T., Carninci, P., Kawai, J., Hayashizaki, Y., Kinoshita, K., Güntert, P., Kigawa, T. & Yokoyama, S. Solution structure of the RWD domain of the mouse GCN2 protein[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Nameki04a.pdf .] [http://dx.doi.org/10.1110/ps.04751804 Protein Sci. 13, 2089–2100 (2004)] | |Nameki, N., Yoneyama, M., Koshiba, S., Tochio, N., Inoue, M., Seki, E., Matsuda, T., Tomo, Y., Harada, T., Saito, K., Kobayashi, N., Yabuki, T., Aoki, M., Nunokawa, E., Matsuda, N., Sakagami, N., Terada, T., Shirouzu, M., Yoshida, M., Hirota, H., Osanai, T., Tanaka, A., Arakawa, T., Carninci, P., Kawai, J., Hayashizaki, Y., Kinoshita, K., Güntert, P., Kigawa, T. & Yokoyama, S. Solution structure of the RWD domain of the mouse GCN2 protein[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Nameki04a.pdf .] [http://dx.doi.org/10.1110/ps.04751804 Protein Sci. 13, 2089–2100 (2004)] | ||
| Line 198: | Line 198: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1q9g asym r 250.jpg|120px]] |
|Fernández, C., Hilty, C., Wider, G., Güntert, P. & Wüthrich, K. NMR structure of the integral membrane protein OmpX[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Fernandez04.pdf .] [http://dx.doi.org/10.1016/j.jmb.2003.09.014 J. Mol. Biol. 336, 1211–1221 (2004)] | |Fernández, C., Hilty, C., Wider, G., Güntert, P. & Wüthrich, K. NMR structure of the integral membrane protein OmpX[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Fernandez04.pdf .] [http://dx.doi.org/10.1016/j.jmb.2003.09.014 J. Mol. Biol. 336, 1211–1221 (2004)] | ||
| Line 204: | Line 204: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1wln asym r 250.jpg|120px]] |
|FHA domain of mouse afadin 6. | |FHA domain of mouse afadin 6. | ||
| Line 210: | Line 210: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1pbu asym r 250.jpg|120px]] |
|Vanwetswinkel, S., Kriek, J., Andersen, G. R., Güntert, P., Dijk, P., Canters, G. W. & Siegal, G. Solution structure of the 162 residue C-terminal domain of human elongation factor 1Bγ[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Vanwetswinkel03.pdf .] [http://www.jbc.org/cgi/content/short/278/44/43443 J. Biol. Chem. 278, 43443–43451 (2003)] | |Vanwetswinkel, S., Kriek, J., Andersen, G. R., Güntert, P., Dijk, P., Canters, G. W. & Siegal, G. Solution structure of the 162 residue C-terminal domain of human elongation factor 1Bγ[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Vanwetswinkel03.pdf .] [http://www.jbc.org/cgi/content/short/278/44/43443 J. Biol. Chem. 278, 43443–43451 (2003)] | ||
| Line 216: | Line 216: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1mo7 asym r 250.jpg|120px]] |
|Hilge, M., Siegal, G., Vuister, G. W., Güntert, P., Gloor, S. M. & Abrahams, J. P. ATP-induced conformational changes of the nucleotide-binding domain of Na,K-ATPase[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Hilge03.pdf .] [Nat. Struct. Biol. 10, 468–474 (2003)] | |Hilge, M., Siegal, G., Vuister, G. W., Güntert, P., Gloor, S. M. & Abrahams, J. P. ATP-induced conformational changes of the nucleotide-binding domain of Na,K-ATPase[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Hilge03.pdf .] [Nat. Struct. Biol. 10, 468–474 (2003)] | ||
| Line 222: | Line 222: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1lg4 asym r 250.jpg|120px]] |
|Lührs, T., Riek, R., Güntert, P. & Wüthrich, K. NMR structure of the human doppel protein[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Luhrs03.pdf .] [http://dx.doi.org/10.1016/S0022-2836(02)01471-7 J. Mol. Biol. 326, 1549–1557 (2003)] | |Lührs, T., Riek, R., Güntert, P. & Wüthrich, K. NMR structure of the human doppel protein[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Luhrs03.pdf .] [http://dx.doi.org/10.1016/S0022-2836(02)01471-7 J. Mol. Biol. 326, 1549–1557 (2003)] | ||
| Line 228: | Line 228: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1h0l asym r 250.jpg|120px]] |
|Zahn, R., Güntert, P. & Wüthrich, K. NMR structure of a variant human prion protein with two disulfide bonds[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Zahn03.pdf .] [http://dx.doi.org/10.1016/S0022-2836(02)01332-3 J. Mol. Biol. 326, 225–234 (2003)] | |Zahn, R., Güntert, P. & Wüthrich, K. NMR structure of a variant human prion protein with two disulfide bonds[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Zahn03.pdf .] [http://dx.doi.org/10.1016/S0022-2836(02)01332-3 J. Mol. Biol. 326, 225–234 (2003)] | ||
| Line 234: | Line 234: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1sr3 asym r 250.jpg|120px]] |
|Enggist, E., Thöny-Meyer, L., Güntert, P. & Pervushin, K. NMR structure of the heme chaperone CcmE reveals a new functional motif[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Enggist02.pdf .] [http://dx.doi.org/10.1016/S0969-2126(02)00885-7 Structure 10, 1551–1557 (2002)] | |Enggist, E., Thöny-Meyer, L., Güntert, P. & Pervushin, K. NMR structure of the heme chaperone CcmE reveals a new functional motif[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Enggist02.pdf .] [http://dx.doi.org/10.1016/S0969-2126(02)00885-7 Structure 10, 1551–1557 (2002)] | ||
| Line 240: | Line 240: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1ls8 asym r 250.jpg|120px]] |
|Lee, D., Damberger, F. D., Peng, G., Horst, R., Güntert, P., Nikonova, L., Leal, W. S. & Wüthrich, K. NMR structure of the unliganded ''Bombyx mori'' pheromone-binding protein at physiological pH[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Lee02.pdf .] [http://dx.doi.org/10.1016/S0014-5793(02)03548-2 FEBS Lett. 531, 314–318 (2002)] | |Lee, D., Damberger, F. D., Peng, G., Horst, R., Güntert, P., Nikonova, L., Leal, W. S. & Wüthrich, K. NMR structure of the unliganded ''Bombyx mori'' pheromone-binding protein at physiological pH[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Lee02.pdf .] [http://dx.doi.org/10.1016/S0014-5793(02)03548-2 FEBS Lett. 531, 314–318 (2002)] | ||
| Line 246: | Line 246: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1k9c asym r 250.jpg|120px]] |
|Ellgaard, L., Bettendorff, P., Braun, D., Herrmann, T., Fiorito, F., Jelesarov, I., Güntert, P., Helenius, A. & Wüthrich, K. NMR Structures of 36 and 73-residue fragments of the calreticulin P-domain[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Ellgaard02.pdf .] [http://dx.doi.org/10.1016/S0022-2836(02)00812-4 J. Mol. Biol. 322, 773–784 (2002)] | |Ellgaard, L., Bettendorff, P., Braun, D., Herrmann, T., Fiorito, F., Jelesarov, I., Güntert, P., Helenius, A. & Wüthrich, K. NMR Structures of 36 and 73-residue fragments of the calreticulin P-domain[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Ellgaard02.pdf .] [http://dx.doi.org/10.1016/S0022-2836(02)00812-4 J. Mol. Biol. 322, 773–784 (2002)] | ||
| Line 252: | Line 252: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1jas asym r 250.jpg|120px]] |
|Miura, T., Klaus, W., Ross, A., Güntert, P., Senn, H. The NMR structure of the class I human ubiquitin-conjugating enzyme 2b[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Miura02.pdf .] [http://dx.doi.org/10.1023/A:1013807519703 J. Biomol. NMR 22, 89–92 (2002)] | |Miura, T., Klaus, W., Ross, A., Güntert, P., Senn, H. The NMR structure of the class I human ubiquitin-conjugating enzyme 2b[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Miura02.pdf .] [http://dx.doi.org/10.1023/A:1013807519703 J. Biomol. NMR 22, 89–92 (2002)] | ||
| Line 258: | Line 258: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1gm0 asym r 250.jpg|120px]] |
|Horst, R., Damberger, F., Luginbühl, P., Güntert, P., Peng, G., Nikonova, L., Leal, W. S. & Wüthrich, K. NMR structure reveals intramolecular regulation mechanism for pheromone binding and release[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Horst01.pdf .] [http://dx.doi.org/10.1073/pnas.251532998 Proc. Natl. Acad. Sci. USA 98, 14374–14379 (2001)] | |Horst, R., Damberger, F., Luginbühl, P., Güntert, P., Peng, G., Nikonova, L., Leal, W. S. & Wüthrich, K. NMR structure reveals intramolecular regulation mechanism for pheromone binding and release[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Horst01.pdf .] [http://dx.doi.org/10.1073/pnas.251532998 Proc. Natl. Acad. Sci. USA 98, 14374–14379 (2001)] | ||
| Line 264: | Line 264: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1hhn asym r 250.jpg|120px]] |
|Ellgaard, L., Riek, R., Herrmann, T., Güntert, P., Braun, D., Helenius, A. & Wüthrich, K. NMR structure of the calreticulin P-domain[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Ellgaard01.pdf .] [http://dx.doi.org/10.1073/pnas.051630098 Proc. Natl. Acad. Sci. USA 98, 3133–3138 (2001)] | |Ellgaard, L., Riek, R., Herrmann, T., Güntert, P., Braun, D., Helenius, A. & Wüthrich, K. NMR structure of the calreticulin P-domain[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Ellgaard01.pdf .] [http://dx.doi.org/10.1073/pnas.051630098 Proc. Natl. Acad. Sci. USA 98, 3133–3138 (2001)] | ||
| Line 270: | Line 270: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1e1g asym r 250.jpg|120px]] |
|Calzolai, L., Lysek, D. A., Güntert, P., von Schroetter, C., Riek, R., Zahn, R. & Wüthrich, K. NMR structures of three single-residue variants of the human prion protein[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Calzolai00.pdf .] [http://www.pnas.org/content/97/15/8340.abstract Proc. Natl. Acad. Sci. USA 97, 8340–8345 (2000)] | |Calzolai, L., Lysek, D. A., Güntert, P., von Schroetter, C., Riek, R., Zahn, R. & Wüthrich, K. NMR structures of three single-residue variants of the human prion protein[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Calzolai00.pdf .] [http://www.pnas.org/content/97/15/8340.abstract Proc. Natl. Acad. Sci. USA 97, 8340–8345 (2000)] | ||
| Line 276: | Line 276: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1qjk asym r 250.jpg|120px]] |
|Riek, R., Prêcheur, B., Wang, Y., Wider, G., Güntert, P., Liu, A., Kägi, J. H. R. & Wüthrich, K. NMR structure of the sea urchin (''Strongylocentrotus purpuratus'') metallothionein MTA[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Riek99.pdf .] [http://dx.doi.org/10.1006/jmbi.1999.2967 J. Mol. Biol. 291, 417–428 (1999)] | |Riek, R., Prêcheur, B., Wang, Y., Wider, G., Güntert, P., Liu, A., Kägi, J. H. R. & Wüthrich, K. NMR structure of the sea urchin (''Strongylocentrotus purpuratus'') metallothionein MTA[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Riek99.pdf .] [http://dx.doi.org/10.1006/jmbi.1999.2967 J. Mol. Biol. 291, 417–428 (1999)] | ||
| Line 282: | Line 282: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1bf8 asym r 250.jpg|120px]] |
|Pellecchia, M., Güntert, P., Glockshuber, R. & Wüthrich, K. The NMR solution structure of the periplasmic chaperone FimC[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Pellecchia98b.pdf .] [http://dx.doi.org/10.1038/2325 Nature Struct. Biol. 5, 885–890 (1998)] | |Pellecchia, M., Güntert, P., Glockshuber, R. & Wüthrich, K. The NMR solution structure of the periplasmic chaperone FimC[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Pellecchia98b.pdf .] [http://dx.doi.org/10.1038/2325 Nature Struct. Biol. 5, 885–890 (1998)] | ||
| Line 288: | Line 288: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1oca asym r 250.jpg|120px]] |
|Ottiger, M., Zerbe, O., Güntert, P. & Wüthrich, K. The NMR solution conformation of unligated human Cyclophilin A[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Ottiger97.pdf .] [http://dx.doi.org/10.1006/jmbi.1997.1220 J. Mol. Biol. 272, 64–81 (1997)] | |Ottiger, M., Zerbe, O., Güntert, P. & Wüthrich, K. The NMR solution conformation of unligated human Cyclophilin A[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Ottiger97.pdf .] [http://dx.doi.org/10.1006/jmbi.1997.1220 J. Mol. Biol. 272, 64–81 (1997)] | ||
| Line 294: | Line 294: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1wkt asym r 250.jpg|120px]] |
|Antuch, W., Güntert, P. & Wüthrich, K. Ancestral βγ-crystallin precursor structure in a yeast killer toxin[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Antuch96.pdf .] [http://dx.doi.org/10.1038/nsb0896-662 Nature Struct. Biol. 3, 662–665 (1996)] | |Antuch, W., Güntert, P. & Wüthrich, K. Ancestral βγ-crystallin precursor structure in a yeast killer toxin[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Antuch96.pdf .] [http://dx.doi.org/10.1038/nsb0896-662 Nature Struct. Biol. 3, 662–665 (1996)] | ||
| Line 300: | Line 300: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1tap asym r 250.jpg|120px]] |
|Antuch, W., Güntert, P., Billeter, M., Hawthorne, T., Grossenbacher, H. & Wüthrich, K. NMR solution structure of the recombinant tick anticoagulant protein (rTAP), a factor Xa inhibitor from the tick ''Ornithodoros moubata''[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Antuch94a.pdf .] [http://dx.doi.org/10.1016/0014-5793(94)00941-4 FEBS Lett. 352, 251–257 (1994)] | |Antuch, W., Güntert, P., Billeter, M., Hawthorne, T., Grossenbacher, H. & Wüthrich, K. NMR solution structure of the recombinant tick anticoagulant protein (rTAP), a factor Xa inhibitor from the tick ''Ornithodoros moubata''[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Antuch94a.pdf .] [http://dx.doi.org/10.1016/0014-5793(94)00941-4 FEBS Lett. 352, 251–257 (1994)] | ||
| Line 306: | Line 306: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1dtk asym r 250.jpg|120px]] |
|Berndt, K. D., Güntert, P. & Wüthrich, K. The NMR solution structure of dendrotoxin K from the venom of ''Dendroaspis polylepis polylepis''[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Berndt93-DendrotoxinK.pdf .] [http://dx.doi.org/10.1006/jmbi.1993.1623 J. Mol. Biol. 234, 735–750 (1993)] | |Berndt, K. D., Güntert, P. & Wüthrich, K. The NMR solution structure of dendrotoxin K from the venom of ''Dendroaspis polylepis polylepis''[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Berndt93-DendrotoxinK.pdf .] [http://dx.doi.org/10.1006/jmbi.1993.1623 J. Mol. Biol. 234, 735–750 (1993)] | ||
| Line 312: | Line 312: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1hic asym r 250.jpg|120px]] |
|Szyperski, T., Güntert, P., Stone, S. R. & Wüthrich, K. NMR solution structure of hirudin(1–51) and comparison with corresponding three-dimensional structures determined using the complete 65-residue hirudin polypeptide chain[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Szyperski92-HirudinStructure.pdf .] [http://dx.doi.org/10.1016/0022-2836(92)90325-E J. Mol. Biol. 228, 1193–1205 (1992)] | |Szyperski, T., Güntert, P., Stone, S. R. & Wüthrich, K. NMR solution structure of hirudin(1–51) and comparison with corresponding three-dimensional structures determined using the complete 65-residue hirudin polypeptide chain[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Szyperski92-HirudinStructure.pdf .] [http://dx.doi.org/10.1016/0022-2836(92)90325-E J. Mol. Biol. 228, 1193–1205 (1992)] | ||
| Line 318: | Line 318: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:1pit asym r 250.jpg|120px]] |
|Berndt, K. D., Güntert, P., Orbons, L. P. M. & Wüthrich, K. Determination of a high-quality NMR solution structure of the bovine pancreatic trypsin inhibitor (BPTI) and comparison with three crystal structures[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Berndt92-BPTIStructure.pdf .] [http://dx.doi.org/10.1016/0022-2836(92)90222-6 J. Mol. Biol. 227, 757–775 (1992)] | |Berndt, K. D., Güntert, P., Orbons, L. P. M. & Wüthrich, K. Determination of a high-quality NMR solution structure of the bovine pancreatic trypsin inhibitor (BPTI) and comparison with three crystal structures[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Berndt92-BPTIStructure.pdf .] [http://dx.doi.org/10.1016/0022-2836(92)90222-6 J. Mol. Biol. 227, 757–775 (1992)] | ||
| Line 324: | Line 324: | ||
|- | |- | ||
| − | |[[image: | + | |[[image:2hoa asym r 250.jpg|120px]] |
|Güntert, P., Qian, Y. Q., Otting, G., Müller, M., Gehring, W. J. & Wüthrich K. Structure determination of the Antp(C39S) homeodomain from nuclear magnetic resonance data in solution using a novel strategy for the structure calculation with the programs DIANA, CALIBA, HABAS and GLOMSA[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Guntert91-AntpC39S.pdf .] [http://dx.doi.org/10.1016/0022-2836(91)90755-U J. Mol. Biol. 217, 531–540 (1991)] | |Güntert, P., Qian, Y. Q., Otting, G., Müller, M., Gehring, W. J. & Wüthrich K. Structure determination of the Antp(C39S) homeodomain from nuclear magnetic resonance data in solution using a novel strategy for the structure calculation with the programs DIANA, CALIBA, HABAS and GLOMSA[http://www.bpc.uni-frankfurt.de/guentert/Intranet/Reprints/Guntert91-AntpC39S.pdf .] [http://dx.doi.org/10.1016/0022-2836(91)90755-U J. Mol. Biol. 217, 531–540 (1991)] | ||
Revision as of 12:36, 6 February 2009
NMR protein structures co-authored by P. Güntert.
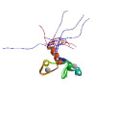
|
He, F., Dang, W., Abe, C., Tsuda, K., Inoue, M., Watanabe, S., Kobayashi, N., Kigawa, T, Matsuda, T., Yabuki, T., Aoki, M., Seki, E., Harada, T., Tomabechi, Y., Terada, T., Shirouzu, M., Tanaka, A., Güntert, P., Muto, Y. & Yokoyama, S. Solution structure of the RNA binding domain in the human muscleblind-like protein 2. Protein Sci. 18, 80-91 (2009)
PDB 2E5S |
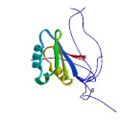
|
Ohnishi, S. Pääkkönen, K., Koshiba, S., Tochio, N., Sato, M., Kobayashi, N., Harada, T., Watanabe, S., Muto, Y., Güntert, P., Tanaka, A., Kigawa, T. & Yokoyama, S. Solution structure of the GUCT domain from human RNA helicase II/Guβ reveals the RRM fold, but implausible RNA interactions. Proteins 74, 133–144 (2009)
PDB 2E29 |
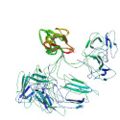
|
Takeda, M., Sugimori, N., Torizawa, T., Terauchi, T., Ono, A. M., Yagi, H., Yamaguchi, Y., Kato, K., Ikeya, T., Jee, J., Güntert, P., Aceti, D. J., Markley, J. L. & Kainosho, M. Structure of the putative 32 kDa myrosinase binding protein from Arabidopsis (At3g16450.1) as determined by the SAIL-NMR method. FEBS J. 275, 5873–5884 (2008)
PDB 2JZ4 NMR restraints BMRB 15607 |
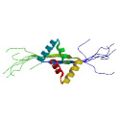
|
Yoshida, H., Furuya, N., Lin, Y. J., Güntert, P., Komano, T. & Kainosho, M. Structural basis of the role of the NikA ribbon-helix-helix domain in initiating bacterial conjugation. J. Mol. Biol. 384, 690–701 (2008) |
|
Lin, Y. J., Umehara, T., Inoue, M., Saito, K., Kigawa, T., Jang, M. K., Ozato, K., Yokoyama, S., Padmanabhan, B., Güntert, P. Solution structure of the extraterminal domain of the bromodomain-containing protein BRD4. Protein Sci. 17, 2174–2179 (2008) |
|
Koglin, A., Löhr, F., Bernhard, F., Rogov, V. R., Frueh, D. P., Strieter, E. R., Mofid, M. R., Güntert, P., Wagner, G., Walsh, C. T., Marahiel, M. A. & Dötsch, V. Structural basis for the selectivity of the external thioesterase of the surfactin-synthetase. Nature 454, 907–911 (2008)
PDB 2RON NMR restraints (SrfTEII) |
|
Nagata, T., Suzuki, S., Endo, R., Shirouzu, M., Terada, T., Inoue, M., Kigawa, T, Güntert, P., Hayashizaki, Y., Muto, Y. & Yokoyama, S. The RRM domain of poly(A)-specific ribonuclease (PARN) has a non-canonical binding site for mRNA cap analog recognition. Nucl. Acids Res. 36, 4754–4767 (2008) |
|
Takeda, M., Chang, C. K., Ikeya, T., Güntert, P., Chang, Y. H., Hsu, Y. L., Huang, T. H. & Kainosho, M. Solution structure of the C-terminal dimerization domain of SARS coronavirus nucleocapsid protein determined by the SAIL-NMR method. J. Mol. Biol. 380, 608–622 (2008) |
|
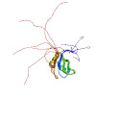
Kuwasako, K., Takahashi, M., Tochio, N., Abe, C., Tsuda, K., Inoue, M., Terada, T., Shirouzu, M., Kobayashi, N., Kigawa, T., Taguchi, S., Tanaka, A., Hayashizaki, Y., Güntert, P., Muto, Y. & Yokoyama, S. Solution structure of the second RNA recognition motif (RRM) domain of murine T cell intracellular antigen-1 (TIA-1) and its RNA recognition mode. Biochemistry 47, 6437–6450 (2008)
PDB 2RNE |
|
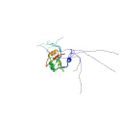
Kuwasako, K., Dohmae, N., Inoue, M., Shirouzu, M., Taguchi, S., Güntert, P., Séraphin, B., Muto, Y. & Yokoyama, S. Complex assembly mechanism and an RNA-binding mode of the human p14-SF3b155 spliceosomal protein complex identified by NMR solution structure and functional analyses. Proteins 71, 1617–1636 (2008)
PDB 2FHO NMR restraints |

|
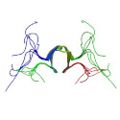
Hwang, E., Ryu, K. S., Pääkkönen, K., Güntert, P., Cheong, H. K., Lim, D. S., Lee, J. O., Jeon, Y. H. & Cheong, C. Structural insight into dimeric interaction of the SARAH domains from Mst1 and RASSF family proteins in the apoptosis pathway. Proc. Natl. Acad. Sci. USA. 104, 9236–9241 (2007)
PDB 2JO8 NMR restraints |
|
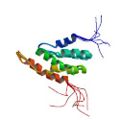
Ohnishi, S. Güntert, P., Koshiba, S., Tomizawa, T., Akasaka, R., Tochio, N., Sato, M., Inoue, M., Harada, T., Watanabe, S., Tanaka, T., Shirouzu, M., Kigawa, T. & Yokoyama, S. Solution structure of an atypical WW domain in a novel β-clam-like dimeric form. FEBS Lett. 581, 462–468 (2007)
PDB 2DWV |

|
Kuwasako, K., He, F., Inoue, M., Tanaka, A., Sugano, S., Güntert, P., Muto, Y. & Yokoyama, S. Solution structures of the SURP domains and the subunit-assembly mechanism within the splicing factor SF3a complex in 17S U2 snRNP. Structure 14, 1677–1689 (2006) |
|
ENTH-VHS domain At3g16270(9-135) from Arabidopsis thaliana.
López-Méndez, B. & Güntert, P. Automated protein structure determination from NMR spectra. J. Am. Chem. Soc. 128, 13112–13122 (2006) PDB 2DCP NMR restraints Note: Automated structure determination with FLYA. Structure does not supersede the original deposition 1VDY. |

|
Rhodanese homology domain At3g16270(175-295) from Arabidopsis thaliana.
López-Méndez, B. & Güntert, P. Automated protein structure determination from NMR spectra. J. Am. Chem. Soc. 128, 13112–13122 (2006) PDB 2DCQ NMR restraints Note: Automated structure determination with FLYA. Structure does not supersede the original deposition 1VEE. |

|
Src homology domain 2 from the human feline sarcoma oncogene Fes.
López-Méndez, B. & Güntert, P. Automated protein structure determination from NMR spectra. J. Am. Chem. Soc. 128, 13112–13122 (2006) PDB 2DCR NMR restraints |
| 120px | Jurt, S., Aemissegger, A., Güntert, P., Zerbe, O. & Hilvert, D. A photoswitchable miniprotein based on the sequence of avian pancreatic polypeptide. Angew. Chem. Int. Ed. 45, 6297-6300 (2006)
PDB 2H4B NMR restraints (cis-1-PP) |
| 120px | Hamada, T., Ito, Y., Abe, T., Hayashi, F., Güntert, P., Inoue, M., Kigawa, T., Terada, T., Shirouzu, M., Yoshida, M., Tanaka, A., Sugano, S., Yokoyama, S. & Hirota, H. Solution structure of the antifreeze-like domain of human sialic acid synthase. Protein Sci. 15, 1010–1016 (2006)
PDB 1WVO NMR restraints |

|
Aachmann, F. L., Svanem, B. I. G., Güntert, P., Petersen, S. B., Valla, S. & Wimmer, R. NMR structure of the R-module - A parallel β-roll subunit from an Azotobacter vinelandii mannuronan C-5 epimerase. J. Biol. Chem. 281, 7350–7356 (2006)
PDB 2AGM NMR restraints |
|
Stereo-array isotope labeled (SAIL) maltodextrin-binding protein MBP.
Kainosho, M., Torizawa, T., Iwashita, Y., Terauchi, T., Ono, A. M. & Güntert, P. Optimal isotope labeling for NMR protein structure determinations. Nature 440, 52–57 (2006) |
|
Stereo-array isotope labeled (SAIL) calmodulin.
Kainosho, M., Torizawa, T., Iwashita, Y., Terauchi, T., Ono, A. M. & Güntert, P. Optimal isotope labeling for NMR protein structure determinations. Nature 440, 52–57 (2006) |
|
Li, H., Inoue, M., Yabuki, T., Aoki, M., Seki, E., Matsuda, T., Nunokawa, E., Motoda, Y., Kobayashi, A., Terada, T., Shirouzu, M., Koshiba, S., Lin, Y. J., Güntert, P., Suzuki, H., Hayashizaki, Y., Kigawa, T. & Yokoyama, S. Solution structure of the mouse enhancer of rudimentary protein reveals a novel fold. J. Biomol. NMR 32, 329–334 (2005)
PDB 1WWQ |

|
Scott, A., Pantoja-Uceda, D., Koshiba, S., Inoue, M., Kigawa, T., Terada, T., Shirouzu, M., Tanaka, A., Sugano, S., Yokoyama, S. & Güntert, P. Solution structure of the Src homology 2 domain from the human feline sarcoma oncogene Fes. J. Biomol. NMR. 31, 357–361 (2005) |
|
Nameki, N., Tochio, N., Koshiba, S., Inoue, M., Yabuki, T., Aoki, M., Seki, E., Matsuda, T., Fujikura, Y., Saito, M., Ikari, M., Watanabe, M., Terada, T., Shirouzu, M., Yoshida, M., Hirota, H., Tanaka, A., Hayashizaki, Y., Güntert, P., Kigawa, T. & Yokoyama, S. Solution structure of the PWWP domain of the hepatoma-derived growth factor family. Protein Sci. 14, 756–764 (2005)
PDB 1N27 |
|
Calzolai, L., Lysek, D. A., Pérez, D. R., Güntert, P. & Wüthrich, K. Prion protein NMR structures of chicken, turtle and frog. Proc. Natl. Acad. Sci. USA 102, 651–655 (2005)
PDB 1U3M NMR restraints BMRB 6269 (chicken PrP(121-225)) |
|
Lysek, D. A., Schorn, C., Nivon, L. G., Esteve-Moya, V., Christen, B., Calzolai, L., von Schroetter, C., Fiorito, F., Herrmann, T., Güntert, P. & Wüthrich, K. Prion protein NMR structures of cat, dog, pig and sheep. Proc. Natl. Acad. Sci. USA 102, 640–645 (2005)
PDB 1XYJ NMR restraints BMRB 6377 (cat PrP(121-231)) |

|
Pääkkönen, K., Tossavainen, H., Permi, P., Rakkolainen, H., Rauvala, H., Raulo, E., Kilpeläinen, I. & Güntert, P. Solution structures of the first and fourth TSR domains of F-spondin. Proteins 64, 665–672 (2006) |
|
ENTH-VHS domain At3g16270 from Arabidopsis thaliana. |
|
Iwai, H., Forrer, P., Plückthun, A. & Güntert, P. NMR solution structure of the monomeric form of the bacteriophage λ capsid stabilizing protein gpD. J. Biomol. NMR. 31, 351–356 (2005) |
|
Pantoja-Uceda, D., López-Méndez, B., Koshiba, S., Inoue, M., Kigawa, T., Terada, T., Shirouzu, M., Tanaka, A., Seki, M., Shinozaki, K., Yokoyama, S. & Güntert, P. Solution structure of the rhodanese homology domain At4g01050(175–295) from Arabidopsis thaliana. Protein Sci. 14, 224–230 (2005) |
|
Nameki, N., Yoneyama, M., Koshiba, S., Tochio, N., Inoue, M., Seki, E., Matsuda, T., Tomo, Y., Harada, T., Saito, K., Kobayashi, N., Yabuki, T., Aoki, M., Nunokawa, E., Matsuda, N., Sakagami, N., Terada, T., Shirouzu, M., Yoshida, M., Hirota, H., Osanai, T., Tanaka, A., Arakawa, T., Carninci, P., Kawai, J., Hayashizaki, Y., Kinoshita, K., Güntert, P., Kigawa, T. & Yokoyama, S. Solution structure of the RWD domain of the mouse GCN2 protein. Protein Sci. 13, 2089–2100 (2004) |

|
Fernández, C., Hilty, C., Wider, G., Güntert, P. & Wüthrich, K. NMR structure of the integral membrane protein OmpX. J. Mol. Biol. 336, 1211–1221 (2004) |
|
FHA domain of mouse afadin 6.
PDB 1WLN |
|
Vanwetswinkel, S., Kriek, J., Andersen, G. R., Güntert, P., Dijk, P., Canters, G. W. & Siegal, G. Solution structure of the 162 residue C-terminal domain of human elongation factor 1Bγ. J. Biol. Chem. 278, 43443–43451 (2003)
PDB 1PBU NMR restraints BMRB 5628 |
| 120px | Hilge, M., Siegal, G., Vuister, G. W., Güntert, P., Gloor, S. M. & Abrahams, J. P. ATP-induced conformational changes of the nucleotide-binding domain of Na,K-ATPase. [Nat. Struct. Biol. 10, 468–474 (2003)]
PDB 1MO7 BMRB 5577 (free form) |

|
Lührs, T., Riek, R., Güntert, P. & Wüthrich, K. NMR structure of the human doppel protein. J. Mol. Biol. 326, 1549–1557 (2003)
PDB 1LG4 NMR restraints BMRB 5145 |
|
Zahn, R., Güntert, P. & Wüthrich, K. NMR structure of a variant human prion protein with two disulfide bonds. J. Mol. Biol. 326, 225–234 (2003)
PDB 1H0L NMR restraints BMRB 5378 |

|
Enggist, E., Thöny-Meyer, L., Güntert, P. & Pervushin, K. NMR structure of the heme chaperone CcmE reveals a new functional motif. Structure 10, 1551–1557 (2002)
PDB 1SR3 NMR restraints |
|
Lee, D., Damberger, F. D., Peng, G., Horst, R., Güntert, P., Nikonova, L., Leal, W. S. & Wüthrich, K. NMR structure of the unliganded Bombyx mori pheromone-binding protein at physiological pH. FEBS Lett. 531, 314–318 (2002)
PDB 1LS8 |
|
Ellgaard, L., Bettendorff, P., Braun, D., Herrmann, T., Fiorito, F., Jelesarov, I., Güntert, P., Helenius, A. & Wüthrich, K. NMR Structures of 36 and 73-residue fragments of the calreticulin P-domain. J. Mol. Biol. 322, 773–784 (2002)
PDB 1K9C NMR restraints BMRB 5204 (residues 189-261) |

|
Miura, T., Klaus, W., Ross, A., Güntert, P., Senn, H. The NMR structure of the class I human ubiquitin-conjugating enzyme 2b. J. Biomol. NMR 22, 89–92 (2002)
PDB 1JAS NMR restraints BMRB 5038 |
|
Horst, R., Damberger, F., Luginbühl, P., Güntert, P., Peng, G., Nikonova, L., Leal, W. S. & Wüthrich, K. NMR structure reveals intramolecular regulation mechanism for pheromone binding and release. Proc. Natl. Acad. Sci. USA 98, 14374–14379 (2001)
PDB 1GM0 NMR restraints BMRB 4849 |

|
Ellgaard, L., Riek, R., Herrmann, T., Güntert, P., Braun, D., Helenius, A. & Wüthrich, K. NMR structure of the calreticulin P-domain. Proc. Natl. Acad. Sci. USA 98, 3133–3138 (2001) |

|
Calzolai, L., Lysek, D. A., Güntert, P., von Schroetter, C., Riek, R., Zahn, R. & Wüthrich, K. NMR structures of three single-residue variants of the human prion protein. Proc. Natl. Acad. Sci. USA 97, 8340–8345 (2000)
PDB 1E1G NMR restraints BMRB 4736 (variant M166V) |

|
Riek, R., Prêcheur, B., Wang, Y., Wider, G., Güntert, P., Liu, A., Kägi, J. H. R. & Wüthrich, K. NMR structure of the sea urchin (Strongylocentrotus purpuratus) metallothionein MTA. J. Mol. Biol. 291, 417–428 (1999)
PDB 1QJK NMR restraints (α domain) |

|
Pellecchia, M., Güntert, P., Glockshuber, R. & Wüthrich, K. The NMR solution structure of the periplasmic chaperone FimC. Nature Struct. Biol. 5, 885–890 (1998)
PDB 1BF8 NMR restraints BMRB 4070 |
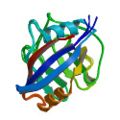
|
Ottiger, M., Zerbe, O., Güntert, P. & Wüthrich, K. The NMR solution conformation of unligated human Cyclophilin A. J. Mol. Biol. 272, 64–81 (1997)
PDB 1OCA NMR restraints |

|
Antuch, W., Güntert, P. & Wüthrich, K. Ancestral βγ-crystallin precursor structure in a yeast killer toxin. Nature Struct. Biol. 3, 662–665 (1996)
PDB 1WKT NMR restraints BMRB [1] |
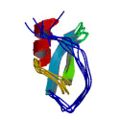
|
Antuch, W., Güntert, P., Billeter, M., Hawthorne, T., Grossenbacher, H. & Wüthrich, K. NMR solution structure of the recombinant tick anticoagulant protein (rTAP), a factor Xa inhibitor from the tick Ornithodoros moubata. FEBS Lett. 352, 251–257 (1994)
PDB 1TAP NMR restraints |

|
Berndt, K. D., Güntert, P. & Wüthrich, K. The NMR solution structure of dendrotoxin K from the venom of Dendroaspis polylepis polylepis. J. Mol. Biol. 234, 735–750 (1993)
PDB 1DTK NMR restraints |
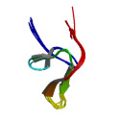
|
Szyperski, T., Güntert, P., Stone, S. R. & Wüthrich, K. NMR solution structure of hirudin(1–51) and comparison with corresponding three-dimensional structures determined using the complete 65-residue hirudin polypeptide chain. J. Mol. Biol. 228, 1193–1205 (1992)
PDB 1HIC NMR restraints |

|
Berndt, K. D., Güntert, P., Orbons, L. P. M. & Wüthrich, K. Determination of a high-quality NMR solution structure of the bovine pancreatic trypsin inhibitor (BPTI) and comparison with three crystal structures. J. Mol. Biol. 227, 757–775 (1992)
PDB 1PIT NMR restraints |

|
Güntert, P., Qian, Y. Q., Otting, G., Müller, M., Gehring, W. J. & Wüthrich K. Structure determination of the Antp(C39S) homeodomain from nuclear magnetic resonance data in solution using a novel strategy for the structure calculation with the programs DIANA, CALIBA, HABAS and GLOMSA. J. Mol. Biol. 217, 531–540 (1991)
PDB 2HOA NMR restraints |